Latent Tuberculosis: Transmission, Diagnosis, Risk factors,
recommendations
Abstract:
In 2017, 10.0 million new cases of
Tuberculosis (TB) disease registered, which involved 5.8 million men, 3.2
million women and 1.0 million children. It’s a global health problem worldwide.
In the same year 1.3 million deaths because of tuberculosis, which making it
one of the top 10 causes of death and the leading cause from a
single infectious agent.
About 1.7 billion people, 23% of the
world’s population, are estimated to have a latent TB infection, and are thus
at risk of developing active TB disease during their lifetime.
Here, we review the transmission occurrence, the
diagnosis and the risk factors of latent tuberculosis, to improve latent
tuberculosis care globally.
Introduction:
In 1882, the Mycobacterium
tuberculosis (MTB) was discovered by Robert Koch. “all who mix with
tuberculosis patient got infected, but remained well so long as they took care
of themselves and kept the soil in condition unfavorable for the growth of the
seed” written by William Osler in 1909[1].
In 2017, 10.0 million new instances
of Tuberculosis (TB) malady enrolled, which included 5.8 million men, 3.2
million ladies, and 1.0 million youngsters. It's a worldwide medical issue.
Around the same time 1.3 million passing in light of tuberculosis, which makes
it one of the main 10 reasons for death and the main source of a solitary
irresistible operator[2].
The start point of the tuberculosis
epidemic is identified from the Latent Mycobacterium Tuberculosis infection
(LTBI). Recently, the worldwide weight of M. Tuberculosis has been re-evaluated
at 24%[3].
About 1.7 billion individuals, 23%
of the total populace, are assessed to have an inactive TB contamination, and
are in this way in danger of creating dynamic TB ailment amid their lifetime,
as indicated by World Health Organization (WHO) [2].
The global rate of decline in
tuberculosis incidence is currently 1.5% and will need to increase to 4% 5% by
2020 and then to 10% per year by 2025 to meet the World Health Organization End
TB Strategy targets (Figure 1) [4]
Tuberculosis transmission:
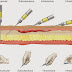
A simple cascade for tuberculosis transmission
is proposed in which a source case of tuberculosis generates infectious
particles, that survive in the air and are inhaled by a susceptible individual,
who may become infected and who then has the potential to develop tuberculosis.
Interventions that target bacterial, host, or behavioral catalysts of
transmission will interrupt tuberculosis transmission and accelerate the
decline in tuberculosis incidence and mortality[5]. Which
summarize in 5 steps (Figure 02):
ü Step 01: source case
ü Step 02: aerosolization
ü Step 03: airborne survival
ü Step 04: exposure and inhalation
ü Step 05: infection
Who transmits the
infection?
The infectiousness of a person with tuberculosis
is higher as well as the degree of smear positivity is increasing, which mean
that the person with smear-positive pulmonary tuberculosis is highly
infectious. In Peru, a large study compared the infectious risk in
smear-positive and smear-negative household, this study showed that person with
smear-positive has a higher risk of infectious, likewise, it found that there is
a low risk of infection in smear-negative persons[6].
However, tuberculosis transmission can also be found in persons with
smear-negative[7]. Coughing is the most efficiency infectious aerosols by shears the
respiratory secretions which contain M, tuberculosis bacteria, from the airways caused by a forced
expiratory maneuver, compared with singing, sneezing, shouting or other respiratory
symptoms[7].
Tuberculosis understanding who are
tainted by HIV, particularly who are in cutting edge immunosuppression, they were
accepted to have less probability at that point patient's HIV-uninfected with
TB to transmit to family unit, presumably in view of a more noteworthy
probability of having smear-negative tuberculosis and a shorter length of
irresistibleness because of increasingly quick movement to death[8, 9].
Site of Transmission
occurrence
In 1905, Robert Koch said in his
Nobel Lecture: “tuberculosis has been called plainly, and quite justly, a
disease of accommodation”[10].
When the age of the household
contact is less than 15 years and the index cases are smear-positive, the
tuberculosis transmission to them is most probably happened[6, 11].
In addition, in high TB burden, in
mines, workplaces, schools, public transportation, and healthcare facilities,
tuberculosis transmission is therefore likely to occur outside the household[9, 11-18].
Person risk
In low and middle incomes countries;
within 95 contact investigation studies, the M. tuberculosis prevalence was
51.5% among contacts. Moreover, the higher risk of tuberculosis infection is HIV
patients and who is <5 years old[19].
Accordingly, the closer contacts
with TB patients the more risk to be infected is higher, which leads to
progress the TB, especially in the first years of exposure, when the infection happens.
[19]
To identify persons with a higher
risk of developing TB, the medical history of the person or a simple test is required[5].
Tuberculosis diagnosis
Tuberculosis skin test
TST is at present still the most
generally utilized and accessible test for the analysis of tuberculous
contamination. Following disease with Mycobacterium tuberculosis, a course of
insusceptible reactions results activated by initiated macrophages and
completed by T cells. Two distinctive cell-intervened insusceptibility systems
result in the two assurances, interceded byTh1 cytokines (interleukin 2,
interleukin 12, and gamma interferon [IFN-γ]), and postponed type excessive
touchiness, intervened by chemokines[20].
Contamination with M. tuberculosis
results in a deferred sort excessive touchiness response to antigens got from
the life form, which is the premise of the TST. Legitimate utilization of the
TST requires information of the antigen utilized, the immunologic reason for
the response to this antigen, the correct procedure of overseeing and perusing
the test, and the consequences of epidemiological and clinical involvement with
the test. At the point when the material is infused intradermally, an exemplary
deferred extreme touchiness response happens in the tainted patient. The
underlying procedure of sharpening following contamination takes around 6 to
about two months, with sharpened T lymphocytes creating in territorial lymph
hubs and entering the dissemination. incitement of these lymphocytes by
intracutaneous infusion of tuberculin results in the indurated skin response of
a positive outcome. The induration is because of cell infiltration interceded by
the sharpened lymphocytes. Thereactionismaximalat48to 72 h and after that
gradually blurs, in spite of the fact that it usually goes on for in excess of
96 h. Two sorts of tuberculin arrangements have been being used, old tuberculin
(OT) and purified protein subordinate (PPD)[20, 21].
TUBERCULIN—HISTORICAL
PERSPECTIVE
Tuberculin was built up 10 years
after Robert Koch found the tubercle bacillus and furthermore a technique for
developing it in unadulterated culture in 1882. He thought of this readiness
from warmth disinfected societies of tubercle bacilli that were filtered and
focused and contained tuberculous proteins. It was at first utilized and touted
as restorative. In any case, the healing estimation of the readiness was
frustrating; in any case, it prompted the disclosure of tuberculin's analytic
esteem. Since the first arrangement, known as OT (old tuberculin), was an
unrefined item with the unessential material present, a positive response came
up short on the affectability to be demonstrative of disease with M.
tuberculosis. OT was accessible for various cut tests[21].
PURIFIED PROTEIN
DERIVATIVE PPD
was initially created by Florence
Siebert in 1939 at the Phipps Institute in Philadelphia, PA. It is an encourage
arranged from filtrates of OT with ammonium sulfate or trichloroacetic corrosive.
The reference standard material for all tuberculin is PPD-S. In 1972, the
Bureau of Biologics of the Food and Drug Administration ordered that the
standard test portion of all Tween-containing PPD tuberculin authorized for use
in people be naturally comparable to 5 tuberculin units (TU) of PPD-S[21, 22].
The definition of tuberculous
infectionisapositivereactionto5TU of PPD[22]. Tween 80 is added to the PPD diluent to keep antigenic material
from being consumed by the glass and plastic holders and syringes, in this
manner anticipating diminished strength of the planning. PPD antigen is
controlled by different cut tests and by the intradermal Mantoux test. Various
cut and PPD qualities of 1 and 250 TU are not exact and are never again
utilized.
MANTOUX TEST
The Mantoux test is performed by
intradermally infusing 0.1 ml of PPD tuberculin (5 TU) into the skin of the
volar part of the lower arm. A solitary portion plastic syringe is utilized
with a 26-to 27-check needle. The infusion is finished with the needle slant
upward. An unmistakable wheal 6 to 10 mm in breadth should result. The best
possible portion is significant; the bigger the portion, the bigger the
response. Flimsier dosages produce littler responses. The test is perused in 48
to 72 h[21].
Test significance relies upon the
nearness or nonappearance of induration. The nearness of induration is
controlled by contact. The distance across of the induration is estimated
transversely. Erythema isn't considered. The span of induration (in
millimeters), the antigen quality and parcel number, the date of testing, and
the date of perusing are altogether recorded. Unfavorable responses to PPD are
strange. Some delicate people may create nearby ulceration or vesicle
arrangement. Fever and lymphadenopathy may likewise happen. Beside neighborhood
wound consideration, no specific treatment is shown in these examples[21].
A new concept of Latent tuberculosis diagnosis
More broadly available methods for
the detection of LTBI include the tuberculin skin test (TST) and the interferon-gamma
release assay (IGRA)[23].
although generally the IGRA is
reported to yield results of higher specificity because of the use of antigens
which are specific to M. tb[24]. The TST, which utilizes antigens shared across mycobacterial
species, suffers from relatively low specificity because of the false-positive
response. of individuals who have been exposed to environmental mycobacteria or
the BCG vaccine [23].
Araujo et al. [25] recently explored the response of newly infected individuals to
several
latency-associated antigens (LAA) over the course of a year, garnering some insights into which host responses may portend progression to active disease. T-cell responsiveness to heparin-binding hemagglutinin (HBHA) has been proposed to predict reactivation risk[26]. In an HIV-positive population, T-cell response to HBHA was negatively correlated with reactivation[27], confirming that a positive T-cell response to HBHA may indicate that a patient will continue to contain the disease.
latency-associated antigens (LAA) over the course of a year, garnering some insights into which host responses may portend progression to active disease. T-cell responsiveness to heparin-binding hemagglutinin (HBHA) has been proposed to predict reactivation risk[26]. In an HIV-positive population, T-cell response to HBHA was negatively correlated with reactivation[27], confirming that a positive T-cell response to HBHA may indicate that a patient will continue to contain the disease.
De Groote et al.[28] also
found higher levels of granzyme B in the blood of LTBI patients,
highlighting the importance of the adaptive immune system in the maintenance of LTBI and pointing to granzyme B as a possible indicator of reduced reactivation risk.
highlighting the importance of the adaptive immune system in the maintenance of LTBI and pointing to granzyme B as a possible indicator of reduced reactivation risk.
Risk factors of Tuberculosis:
HIV coinfection:
Known as the most strong risk factor
of reactivation for TB[29]. According to different studies, HIV infection might lead from 10
to 100 times higher risk of LTB infection reactivation[30-32].
In 2019, the WHO rules on
tuberculosis contamination aversion and control demonstrated the significance
of LTBI treatment in HIV patients in both low-and high-pay nations [33].
Along these lines, the WHO suggested
that all HIV patients who have obscure or positive screening test results and
have no proof of dynamic TB get prophylaxis, despite the fact that patients
with a positive TST or IGRA result may profit more from preventive treatment.
For HIV patients with negative screening test results, doctors ought to assess
their individual TB hazards and choose whether treatment ought to be
recommended[34].
Diabetes mellitus
Patients who diagnosed with diabetes
mellitus (DM) have a higher risk to get an infection from latent to active
tuberculosis[35]. 15%
of tuberculosis patient might be linked to DM.
WHO collaborative framework for TB
and DM recommended bidirectional screening-screening for both all patient who
have Tuberculosis or diabetes mellitus[36].
A study of DM patients gives a
result that when the hemoglobin A1c (HbA1c) level is greater or equal 7% the
risk of active tuberculosis is three times more than those who have HbA1c less
the 7%[37]. Night sweats, dyspnea, fever, and weight loss are the clinical symptoms
in a patient who have DM[37]. Even the patient who use insulin more the 40 units have a higher
risk for TB more than patients use less does.
Usually, female, older and obese
consider as TB patient and diagnosed previously from DM. In the opposite, patients
with TB and newly-diagnosed DM are male and younger, as well as to have a lower
level of HbA1c[38].
Smoking
Tuberculosis rates are higher in
underdeveloped or developing countries, where from 1.3 billion tobacco consumer
worldwide live[39].
Regarding smoking role in
tuberculosis pathogenesis, have a relation to reduced immune response, to
ciliary dysfunction and to defects in the immune response of macrophage rising
the M. tuberculosis infection[39]. Death due TB is nine times higher in smokers compared to never
use tobacco in his life[40].
In 2017, a prospective study in
rural China, confirm that smoking is important to risk factor especially in old
smokers for tuberculosis infection, as well as give strong evidence of the
relation between persons who have smoking history and the risk of talent tuberculosis[41].
Ongoing examinations recommend that
in the recognition of idle tuberculosis with IFN-γ strategies, the extent of
false-negative outcomes is higher among smokers than among nonsmokers, and that
smoking negatively affects the aftereffects of tuberculosis treatment[42].
Drink of Alcohol
Worldwide, alcohol consumption is a
normal habit, which could be addictive to many people. Alcohol is considered as
one of the top 5 risk factors of different diseases and death. It has been a
causal factor in more than 200 diseases in the world[32]. From tuberculosis cases, about 10% are related to alcohol[43].
A partner of people experiencing
liquor use issue was pursued for a long time, In a planned report in New York
City, demonstrated that the occurrence of tuberculosis is multiple times the
frequency analyzed the age-coordinated all-inclusive community[44].
in China, a prospective cohort study
of adults followed for 16.8 ± 5.2 years[45].
The result showed the association between the alcohol consumption and the
rising of tuberculosis risk, which is more have a more higher risk when alcohol
(≥ 2 drinks per day) and smoking are combined together[46].
Illegal Drug Use
The relationship between illegal
drug use and tuberculosis is increasing according to the epidemiological data,
because of the human, economic, social and political aspect it leads to a
public health problem[47].
The utilization of powder or rocks
was found, in two separate studies [48, 49], to
be connected legitimately with the predominance of dynamic and inactive
tuberculosis; delays in the higher rates of retreatment; and the development of
multidrug-safe strains; conclusion of the malady; resistance with and surrender
of treatment.
In the US, an investigation of 147
inpatients which finding of mental sicknesses, demonstrated that the
utilization of rocks has an association with a positive PPD skin test. The
hazard in medication clients is higher contrasted and non-tranquilize clients[50].
There is a big need from the health
professions and the governments around the world to create new strategies and
new policy to control the drug user in the society, because of the increasing
of drug users in many countries, which is correlated with tuberculosis
infection disease[35].
Latent Tuberculosis Infection recommendations
Close contact TB
Population who had a
close contact with drug-susceptible TB infection patients ought to be testing
and treatment should be given as a priority, due to probability of an early
progression of the disease maybe observed. Moreover, more priority must be
applied for the child when a TB patient contact happened[51].
Risk of sever form of
the dieses might be increase particularly in those who are under five years
old. The lethal epidemic on the generic screen has proven to be a reliable,
non-tuberculosis ant tuberculous treatment prior to conventional treatment[52].
Given the logistical
barriers to the use of TST in restricted pores and excellent resistance to
pediatric prophylaxis, an experimental treatment may be useful for newly
established young children. For children the effective treatment involves the
same adult LTBI antibiotics, with a dose-adjusted dose based on weight[53].
The most important
contact group recommended from WHO for treatment is children under 5 years of
age and HIV-infected people, given their susceptibility to the development of
serious diseases. However, these guidelines recognize the importance of
providing preventive care for all infected persons, regardless of age or
associated diseases, as far as possible[51].
New migrants
In areas of low
prevalence, most tuberculosis is common among newly arrived immigrants in the
affected area. To significantly reduce tuberculosis in such an environment, you
must prevent infection of infected immigrants with tuberculosis. Preventive
testing for active tuberculosis is carried out in many environments, including
the United States, Europe, Australia and Canada. However, until recently,
testing for LTBI was not recommended. The CDC concluded that it would provide
corresponding net economic benefits if screening of individuals in selected countries
at high risk for LTBI would be possible[54]. In the UK, new immigrants under the age of 35 are selected in
countries with a TB incidence of 150/100 000[55].
This policy is based on the assumption that the toxicity of
treatment will be relatively small compared with the long-term interests of
individuals and groups of the population. Recognizing that most local
displacement occurs between recent immigrants; this reflects growth momentum
for comprehensive testing and treatment of LTI in resource-rich countries[56].
Ultimately, eradicating
tuberculosis in countries with low prevalence and large numbers of immigrants
will aggressively reduce tuberculosis outbreaks in high-burden countries[57, 58].
Immunocompromised
The risk of the
resumption of tuberculosis is increased in people with immunosuppressive conditions,
such as poorly controlled diabetes
mellitus[59], chronic renal
failure[60], , or treatment with tumor necrosis
factor (TNF) inhibitors[61].
These risk factors for
medical factors should be taken into account when assessing the risk ratio and
the winning factor of a particular patient with LTBI. Modeling analysis of the
solution has shown that most immune damage to patients benefits from the LTBI
treatment[62].
However, there are some
additional factors that should be considered as a solution for determining the
condition of the immune system depression. In some populations, the accuracy of
the prevalence and MTBN test of LTTBI (especially the ratio of false-negative
results of TSTs and IGRAs) will
have a significant effect on the cost and benefit of the screening program[57].
References
1. TE
Dobbs, M.K., AIDS Therapy E-Book. .
2008, PA: Elsevier: Philadelphia.
2. WHO, Global tuberculosis reporte 2018. 2018, France: Paris.
3. Houben, R.M. and P.J. Dodd, The Global Burden of Latent Tuberculosis
Infection: A Re-estimation Using Mathematical Modelling. PLoS Med, 2016. 13(10): p. e1002152.
4. Churchyard, G., et al., What We Know About Tuberculosis
Transmission: An Overview. J Infect Dis, 2017. 216(suppl_6): p. S629-s635.
5. Dowdy, D.W., et al., Transforming the fight against tuberculosis:
targeting catalysts of transmission. Clin Infect Dis, 2014. 59(8): p. 1123-9.
6. Zelner, J.L., et al., Age-specific risks of tuberculosis infection
from household and community exposures and opportunities for interventions in a
high-burden setting. Am J Epidemiol, 2014. 180(8): p. 853-61.
7. Turner, R.D. and G.H. Bothamley, Cough and the transmission of tuberculosis.
J Infect Dis, 2015. 211(9): p.
1367-72.
8. Huang, C.C., et al., The effect of HIV-related immunosuppression
on the risk of tuberculosis transmission to household contacts. Clin Infect
Dis, 2014. 58(6): p. 765-74.
9. Corbett, E.L., et al., Epidemiology of tuberculosis in a high HIV
prevalence population provided with enhanced diagnosis of symptomatic disease.
PLoS Med, 2007. 4(1): p. e22.
10. Koch, R., The Nobel Prize, in Nobel
Lecture. 2014, Stockholm, Sweden: Nobel Media.
11. Andrews, J.R., et al., Integrating social contact and environmental
data in evaluating tuberculosis transmission in a South African township. J
Infect Dis, 2014. 210(4): p.
597-603.
12. Andrews, J.R., C. Morrow, and R. Wood,
Modeling the role of public
transportation in sustaining tuberculosis transmission in South Africa. Am
J Epidemiol, 2013. 177(6): p.
556-61.
13. Yates, T.A., et al., The transmission of Mycobacterium
tuberculosis in high burden settings. Lancet Infect Dis, 2016. 16(2): p. 227-38.
14. Churchyard, G.J., et al., A trial of mass isoniazid preventive therapy
for tuberculosis control. N Engl J Med, 2014. 370(4): p. 301-10.
15. Telisinghe, L., et al., High tuberculosis prevalence in a South
African prison: the need for routine tuberculosis screening. PLoS One,
2014. 9(1): p. e87262.
16. Johnstone-Robertson, S., et al., Tuberculosis in a South African prison - a
transmission modelling analysis. S Afr Med J, 2011. 101(11): p. 809-13.
17. Escombe, A.R., et al., Tuberculosis transmission risk and infection
control in a hospital emergency department in Lima, Peru. Int J Tuberc Lung
Dis, 2010. 14(9): p. 1120-6.
18. Escombe, A.R., et al., Upper-room ultraviolet light and negative
air ionization to prevent tuberculosis transmission. PLoS Med, 2009. 6(3): p. e43.
19. Fox, G.J., et al., Contact investigation for tuberculosis: a
systematic review and meta-analysis. Eur Respir J, 2013. 41(1): p. 140-56.
20. Orme, I.M. and A.M. Cooper, Cytokine/chemokine cascades in immunity to
tuberculosis. Immunol Today, 1999. 20(7):
p. 307-12.
21. Lardizabal, A.A. and L.B. Reichman, Diagnosis of Latent Tuberculosis Infection.
Microbiol Spectr, 2017. 5(1).
22. American
Thoracic Society. Diagnostic standards and classification of tuberculosis and
other mycobacterial diseases (14th edition). Am Rev Respir Dis, 1981. 123(3): p. 343-58.
23. Mazurek, G.H., et al., Updated guidelines for using Interferon
Gamma Release Assays to detect Mycobacterium tuberculosis infection - United
States, 2010. MMWR Recomm Rep, 2010. 59(Rr-5):
p. 1-25.
24. Pai, M. and M. Behr, Latent Mycobacterium tuberculosis Infection
and Interferon-Gamma Release Assays. Microbiol Spectr, 2016. 4(5).
25. de Araujo, L.S., et al., Profile of interferon-gamma response to
latency-associated and novel in vivo expressed antigens in a cohort of subjects
recently exposed to Mycobacterium tuberculosis. Tuberculosis, 2015. 95(6): p. 751-757.
26. Immunological
Signatures Identifying Different Stages of Latent Mycobacterium tuberculosis
Infection and Discriminating Latent from Active Tuberculosis in Humans.
2015. 6(341).
27. Delogu, G., et al., Lack of Response to HBHA in HIV-Infected
Patients with Latent Tuberculosis Infection. Scand J Immunol, 2016. 84(6): p. 344-352.
28. De Groote, M.A., et al., Highly Multiplexed Proteomic Analysis of
Quantiferon Supernatants To Identify Biomarkers of Latent Tuberculosis
Infection. J Clin Microbiol, 2017. 55(2):
p. 391-402.
29. Antonucci, G., et al., Risk factors for tuberculosis in
HIV-infected persons. A prospective cohort study. The Gruppo Italiano di Studio
Tubercolosi e AIDS (GISTA). Jama, 1995. 274(2): p. 143-8.
30. Horsburgh, C.R., Jr. and E.J. Rubin, Clinical practice. Latent tuberculosis
infection in the United States. N Engl J Med, 2011. 364(15): p. 1441-8.
31. Landry, J. and D. Menzies, Preventive chemotherapy. Where has it got
us? Where to go next? Int J Tuberc Lung Dis, 2008. 12(12): p. 1352-64.
32. WHO, GLOBAL HEALTH RISKS: Mortality and burden of disease attributable to
selected major risks. 2009, Switzerland: Geneva.
33. WHO, WHO guidelines on tuberculosis infection prevention and control.
2019, World Health organization: Geneva.
34. WHO, World Health Organization. 2019.
35. Silva, D.R., et al., Risk factors for tuberculosis: diabetes,
smoking, alcohol use, and the use of other drugs. J Bras Pneumol, 2018. 44(2): p. 145-152.
36. Pizzol, D., et al., Tuberculosis and diabetes: current state and
future perspectives. Trop Med Int Health, 2016. 21(6): p. 694-702.
37. Dooley, K.E. and R.E. Chaisson, Tuberculosis and diabetes mellitus:
convergence of two epidemics. Lancet Infect Dis, 2009. 9(12): p. 737-46.
38. Restrepo, B.I., Diabetes and Tuberculosis. Microbiol Spectr, 2016. 4(6).
39. van Zyl Smit, R.N., et al., Global lung health: the colliding epidemics
of tuberculosis, tobacco smoking, HIV and COPD. Eur Respir J, 2010. 35(1): p. 27-33.
40. Wen, C.P., et al., The reduction of tuberculosis risks by
smoking cessation. BMC Infect Dis, 2010. 10: p. 156.
41. Zhang, H., et al., A dose-response relationship of smoking with
tuberculosis infection: A cross-sectional study among 21008 rural residents in
China. PLoS One, 2017. 12(4): p.
e0175183.
42. Altet, N., et al., Assessment of the influence of direct
tobacco smoke on infection and active TB management. PLoS One, 2017. 12(8): p. e0182998.
43. Rehm, J., et al., The association between alcohol use, alcohol use disorders and
tuberculosis (TB). A systematic review. BMC Public Health, 2009. 9: p. 450.
44. Friedman, L.N., et al., Tuberculosis, AIDS, and death among
substance abusers on welfare in New York City. N Engl J Med, 1996. 334(13): p. 828-33.
45. Soh, A.Z., et al., Alcohol drinking and cigarette smoking in
relation to risk of active tuberculosis: prospective cohort study. BMJ Open
Respir Res, 2017. 4(1): p. e000247.
46. Hermosilla, S., et al., Identifying risk factors associated with
smear positivity of pulmonary tuberculosis in Kazakhstan. PLoS One, 2017. 12(3): p. e0172942.
47. Deiss, R.G., T.C. Rodwell, and R.S.
Garfein, Tuberculosis and illicit drug
use: review and update. Clin Infect Dis, 2009. 48(1): p. 72-82.
48. Paixao, L.M. and E.D. Gontijo, [Profile of notified tuberculosis cases and
factors associated with treatment dropout]. Rev Saude Publica, 2007. 41(2): p. 205-13.
49. Rodrigues, I.L., et al., [Abandonment of tuberculosis treatment among
patients co-infected with TB/HIV]. Rev Esc Enferm USP, 2010. 44(2): p. 383-7.
50. Taubes, T., et al., Crack cocaine and schizophrenia as risk
factors for PPD reactivity in the dually diagnosed. J Addict Dis, 1998. 17(3): p. 63-74.
51. World
Health Organisation, in Guidelines on
the management of latent tuberculosis infection. 2015, WHO.
52. Triasih, R., et al., A prospective evaluation of the
symptom-based screening approach to the management of children who are contacts
of tuberculosis cases. Clin Infect Dis, 2015. 60(1): p. 12-8.
53. Marais, B.J., et al., Screening and preventive therapy for
tuberculosis. Clin Chest Med, 2009. 30(4):
p. 827-46, x.
54. Bibbins-Domingo, K., et al., Screening for Latent Tuberculosis Infection
in Adults: US Preventive Services Task Force Recommendation Statement.
Jama, 2016. 316(9): p. 962-9.
55. Pareek, M., et al., Screening of immigrants in the UK for
imported latent tuberculosis: a multicentre cohort study and cost-effectiveness
analysis. Lancet Infect Dis, 2011. 11(6):
p. 435-44.
56. McBryde, E.S. and J.T. Denholm, Risk of active tuberculosis in immigrants:
effects of age, region of origin and time since arrival in a low-exposure
setting. Med J Aust, 2012. 197(8):
p. 458-61.
57. Fox, G.J., et al., Preventive therapy for latent tuberculosis
infection-the promise and the challenges. Int J Infect Dis, 2017. 56: p. 68-76.
58. Denholm, J.T., E.S. McBryde, and G.V.
Brown, Ethical evaluation of immigration
screening policy for latent tuberculosis infection. Australian and New
Zealand Journal of Public Health, 2012. 36(4):
p. 325-328.
59. Dobler, C.C., J.R. Flack, and G.B.
Marks, Risk of tuberculosis among people
with diabetes mellitus: an Australian nationwide cohort study. BMJ Open,
2012. 2(1): p. e000666.
60. Dobler, C.C., S.P. McDonald, and G.B.
Marks, Risk of tuberculosis in dialysis
patients: a nationwide cohort study. PLoS One, 2011. 6(12): p. e29563.
61. Solovic, I., et al., The risk of tuberculosis related to tumour
necrosis factor antagonist therapies: a TBNET consensus statement. Eur
Respir J, 2010. 36(5): p. 1185-206.
62. Dobler, C.C., A. Martin, and G.B.
Marks, Benefit of treatment of latent
tuberculosis infection in individual patients. Eur Respir J, 2015. 46(5): p. 1397-406.









0 commentaires:
Enregistrer un commentaire